Topics of Interests: Thermodynamics, Heat Transfer, Fluid Mechanics, Energy, Management, Entropy Minimization, Exergy, Health and Diet
Blog Archive
35 - T # 7 - What is relative Humidity ? - Posted by Kambiz Ehsani - 5/16/2018
This is the ratio of the amount of moisture present in the air to the amount it can hold at saturation.
34 - T # 6 - Total heat of Air formula - Posted by Kambiz Ehsani - 5/16/2018
When dealing with changes in both sensible and latent heat such as in heating and humidification or in cooling, use the total heat formula for calculating the heat change in the air which states that:
Total Heat in Btu/hr = Specific Density x 60 min/hr x CFM x Delta H
Total Heat in Btu/hr = 0.075 x 60 x CFM x Delta H
Total Heat in Btu/hr = 4.5 x CFM x Delta H
Delta H = Change in the enthalpy of entering and leaving
Total Heat in Btu/hr = Specific Density x 60 min/hr x CFM x Delta H
Total Heat in Btu/hr = 0.075 x 60 x CFM x Delta H
Total Heat in Btu/hr = 4.5 x CFM x Delta H
Delta H = Change in the enthalpy of entering and leaving
33 - T # 5 - Moisture in the air - Post by Kambiz Ehsani - 5/16/2018
Moisture is always present in the air and has a heat content of its own called Latent Heat.
The latent heat of water vapor added to the sensible heat of dry air gives us the total heat of the quantity of air.
We use a Wet Bulb thermometer to measure enthalpy.
The latent heat of water vapor added to the sensible heat of dry air gives us the total heat of the quantity of air.
We use a Wet Bulb thermometer to measure enthalpy.
32 - T # 4 - Air Sensible Heat Formula - Posted by Kambiz Ehsani - 5/16/2018
Sensible Heat of Air = Btu/hr = Sp. Heat x Sp. Gravity x 60 Min/Hr x CFM x Delta T
Sensible Heat of Air = 0.24 x 0.075 x 60 x CFM x Delta T
Sensible Heat of Air = 1.08 x CFM x Delta T
Temperature readings are Dry Bulb
Sensible Heat of Air = 0.24 x 0.075 x 60 x CFM x Delta T
Sensible Heat of Air = 1.08 x CFM x Delta T
Temperature readings are Dry Bulb
31 - T # 3 - Air Properties - Posted by Kambiz Ehsani - 5/16/2018
What is specific heat of air?
Looking at the ability of air to get hot. It is measured in the amount of heat in Btu's required to raise one (1) pound of a substance one (1) degree F. Standard air has a specific heat of 0.24 Btu's per pound per degree F.
Looking at the ability of air to get hot. It is measured in the amount of heat in Btu's required to raise one (1) pound of a substance one (1) degree F. Standard air has a specific heat of 0.24 Btu's per pound per degree F.
30 - Air Properties - T # 2 - Posted by Kambiz Ehsani - 5/16/2018
As Air is heated, It expands. the specific volume would increase and the Specific density would decrease. At higher temperatures, air weighs less per cubic foot of volumes. This is why warm air rises. Example is the balloons that go up with passengers.
29 - Fundamentals - Posted by Kambiz Ehsani - 5/15/2018
How much pressure is a column of water 1 foot high exerting at the base of this column?
Solution:
The answer is 0.433 psi. So 119 psi pressure is equal to 119 / 0.433 = 275 feet
Solution:
The answer is 0.433 psi. So 119 psi pressure is equal to 119 / 0.433 = 275 feet
28 - Air Properties T #1 - Posted by Kambiz Ehsani - 5/15/2018
- Air is a mixture of many gasses and is classified as either Dry or Moist air
- Dry air contains approx. 78% nitrogen , 20.9% Oxygen, 1% Argon and other gasses (1% other gasses
- Moist air is a mixture of dry air and water vapor. The amount of moisture present in the mixture may vary from zero to all that air can hold, called its saturation point.
- If dry air and water vapor were mixed together in a container, the pressure of this mixture is the summation of the pressure exerted by each of the two gasses. This is known as Dalton's Law.
- Dry air is a gas and follow the gas laws of Charles and Boyle. This gas law states that if air were heated and maintained at a constant pressure, the air would expand and weigh less per cubic foot of volume.This property of air is known as specific density.
- At sea level, the pressure exerted on the surface of the mercury of 70 degree dry air is 14.696 psi absolute. This will maintain a level of mercury in the tube at 29.92 inches.
- Specific Density equals one over the specific volume. Or, Specific volume equals one over the specific density. For example, the specific volume of standard air is 13.33 cubic feet per pound, so its density will be one over 13.33 or 0.075 lb per cubic foot.
27 - Health recommendation pick for the week - Benefits of antioxidants - Posted by Kambiz Ehsani - 5/13/2018
Benefits of antioxidants:
https://www.webmd.com/food-recipes/antioxidants-your-immune-system-super-foods-optimal-health
https://www.webmd.com/food-recipes/antioxidants-your-immune-system-super-foods-optimal-health
26 - Question 20 - Boiler Deaerator energy balance - Post by Kambiz Ehsani - 5/12/2018
A boiler generates 50,000 lb/hr of saturated steam at 300
psia, out of which 10,000 lb/hr is taken for process and is returned to the deaerator
as condensate at 180 degree F, the remainder is consumed. Makeup water enters
the DA tank at 70 degree F and steam is available at 300 psia for deaeration.
The DA tank operates at 25 psia. The blow down has TDS of 1,500 ppm by weight
and make up has a TDS of 100 ppm. Evaluate the blow down and deaeration steam
quantities.
Solution:
If boiler concentration is 0.5 ppm solid and water hardness
concentration is 2,500 ppm: then the percent moisture in steam = Steam Purity,
ppm / Boiler water concentration, ppm or
percent moisture in steam = (0.5 / 2,500) * 100 = 0.02 so
the steam quality = 100 – 0.02 = 99.98 %
DA Energy balance:
The mass balance around two control volumes gives us:
If M is the make up
water to DA, D is the steam for deaeration to DA, F the BFW and B is the
Blowdown
For the boiler 50,000 + B = F and for DA: 10,000 + M + D = F
Eq (1)
by equating this two we will have: 50,000 + B = 10,000 + M +
D
10,000 * 148 + 1,202.8 * D + M * 38 = 209 * F = 209 *
(50,000 + B) Eq (2)
Balance of the solids in the makeup and blowdown gives 100 *
M = 1,500 B Eq (3)
25 - Question 19 - General Physics - gym lifting energy burned - Post by Kambiz Ehsani - 5/9/2018 - Solved
You are at gym and working out. You are lifting weight. What is the work for lifting 30 pounds weight for 1 foot? How much energy or calories will you burn if you do this exercise for 10 minutes and you do it 500 times? Although it looks impossible :-)
Solution:
Work for lifting a weight @ gym :
30 lbf lifted 1 ft = 30 * 1 = 30 lbf-ft = 30 * 1.3558 = 41 J
Total work including 500 times lifting = 41 * 500 = 20,500 J
Power = 34 J/sec = Watts or 5 kcal
Assuming the human body efficiency in converting the Fat to exercise of 0.25
Power converted to to burned fat = 5 / 0.75 = approximately 7 Kcal
Solution:
Work for lifting a weight @ gym :
30 lbf lifted 1 ft = 30 * 1 = 30 lbf-ft = 30 * 1.3558 = 41 J
Total work including 500 times lifting = 41 * 500 = 20,500 J
Power = 34 J/sec = Watts or 5 kcal
Assuming the human body efficiency in converting the Fat to exercise of 0.25
Power converted to to burned fat = 5 / 0.75 = approximately 7 Kcal
24 - Question 18 - Entropy Calculation - posted by Kambiz Ehsani - 5/8/2018
A peice of metal with the weight of 100 kg and temperature of 500 K falls into a big lake and gets cooled down with the lake temperature of 285 K. Find the entropy change of the lake water.
23 - Question 17 - Carnot engine - Posted by Kambiz Ehsani - 5/5/2018 - Solved
Consider three carnot machines, where first one works between Th and Tl, second one works between Ti and Tc the third one between Th and Tc. Find the relationship between the efficiencies of the three carnot engines (Eff 1, Eff 2 and Eff 3):
Solution:
Efficiency of the Carnot machine is a function of Tl/Th ;
Efficiency of the First machine = Eff 1 = Tl / Th
Efficiency of the First machine = Eff 2 = Tc / Tl
Efficiency of the First machine = Eff 3 = Tc / Th
Therefore :
Eff 3 = Eff 1 * Eff 2
Solution:
Efficiency of the Carnot machine is a function of Tl/Th ;
Efficiency of the First machine = Eff 1 = Tl / Th
Efficiency of the First machine = Eff 2 = Tc / Tl
Efficiency of the First machine = Eff 3 = Tc / Th
Therefore :
Eff 3 = Eff 1 * Eff 2
22 - Question 16 - Braking Standard Fuel Consumption (BSFC) - Posted by Kambiz Ehsani - 5/5/2018 - Solved
If the thermal efficiency of an engine equals 25% and the heating value of the fuel 36,000 kJ/kg, calculate the Braking Standard Fuel Consumption (BSFC) in kgkW-hr.
Solution:
W = 0.25 * 36,000 = 9,000 kJ/Kg fuel
1 kW - hr = 1 kJ /sec * 3,600 Sec = 3,600 kJ
Therefore the BSFC = 0.4 kg/kW-hr
Solution:
W = 0.25 * 36,000 = 9,000 kJ/Kg fuel
1 kW - hr = 1 kJ /sec * 3,600 Sec = 3,600 kJ
Therefore the BSFC = 0.4 kg/kW-hr
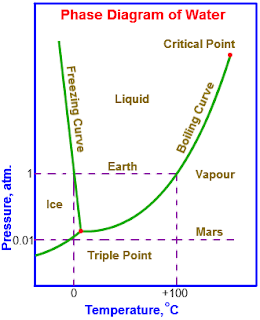
21 - Question 15 - The triple point of the water - Posted by Kambiz Ehsani - 5/5/2018 - Solved
The temperature of the objects on a winter night reaches zero degree C. The moisture in the air is relatively high. Explain which one of the options below is the right process for the moisture on the objects phase change?
1) From vapor to solid
2) From vapor to solid to liquid
3) From vapor to liquid
4) From vapor to liquid to solid
Solution:
Because the temperature of the objects on the surface are below the temperature of the triple point of the water the vapor will become solid directly.
1) From vapor to solid
2) From vapor to solid to liquid
3) From vapor to liquid
4) From vapor to liquid to solid
Solution:
Because the temperature of the objects on the surface are below the temperature of the triple point of the water the vapor will become solid directly.
Photo source :
https://www.quora.com/What-is-the-meaning-of-triple-point-of-water
20 - Question 14 - The Second Law and Carnot Engine - Posted by Kambiz Ehsani - 5/5/2018 - Solved
Is this possible to build a heat engine that works between 800 and 300 degree K and the heat transfer from the hot source Qh = 500 kJ , Ql = 187.5 kJ and produce 200 kJ? Explain your answer.
Solution:
NO beacuse: the First Law is not met here.
Qh - Ql must be the W => this becomes 500 - 187.5 which is not equal the 200 given in the assumptions of the problem and based on the First Law this is not possible because the delta of the Q must be equal to the work created on the shaft and in this example it is not the case.
Solution:
NO beacuse: the First Law is not met here.
Qh - Ql must be the W => this becomes 500 - 187.5 which is not equal the 200 given in the assumptions of the problem and based on the First Law this is not possible because the delta of the Q must be equal to the work created on the shaft and in this example it is not the case.
19 - Question 13 - Thermodynamics - Post by Kambiz Ehsani - 5/5/2018 - Solved
Consider a balloon with the initial internal pressure of P1 when the outside pressure is P0. If air is pumped so that the internal balloon pressure becomes P2, Find the work of system on the surrounding.
Solution:
Assuming the air is the ideal gas and neglect the heat transfer. Therefore the Pv = m RT, since the P is not changing, the work will be applied to increase the volume from V1 to V2 therefore the work will be :
1W2 = P0 (V2 -V1)
Solution:
Assuming the air is the ideal gas and neglect the heat transfer. Therefore the Pv = m RT, since the P is not changing, the work will be applied to increase the volume from V1 to V2 therefore the work will be :
1W2 = P0 (V2 -V1)
18 - What is the Carnot Cycle? Post by Kambiz Ehsani - 5/4/2018 - Solved
Per Wikipedia:"The Carnot cycle is a theoretical thermodynamic cycle proposed by French physicist Sadi Carnot in 1824 and expanded upon by others in the 1830s and 1840s. It provides an upper limit on the efficiency that any classical thermodynamic engine can achieve during the conversion of heat into work, or conversely, the efficiency of a refrigeration system in creating a temperature difference (e.g. refrigeration) by the application of work to the system. It is not an actual thermodynamic cycle but is a theoretical construct."
Why is this cycle so important to design engineers? The Carnot cycle is a theoretical cycle that gives the maximum thermal efficiencies when the cycle works between two thermal sources (high and low temperatures). For example if the Rankine cycle is considered for the argument, where it operates between the temperature of the boiler feed water at 40 and Steam temperature at 400 degree C. What is the Carnot efficiency of such cycle?
Solution:
Efficiency = 1- Tl / Th = 1- (278/478) = 41% and we know that the thermal efficiency of the Rankine Cycle doesn't exceed high 30%. So Rankine Cycle is a high ceiling for the performance or efficiency of a real cycle.
Why is this cycle so important to design engineers? The Carnot cycle is a theoretical cycle that gives the maximum thermal efficiencies when the cycle works between two thermal sources (high and low temperatures). For example if the Rankine cycle is considered for the argument, where it operates between the temperature of the boiler feed water at 40 and Steam temperature at 400 degree C. What is the Carnot efficiency of such cycle?
Solution:
Efficiency = 1- Tl / Th = 1- (278/478) = 41% and we know that the thermal efficiency of the Rankine Cycle doesn't exceed high 30%. So Rankine Cycle is a high ceiling for the performance or efficiency of a real cycle.
17 - Introduction of a book - Shape and Structure, From Engineering to Nature by Adrian Bejan - 5/4/2018
In this book Dr. Bejan propose a topic that has always been and always will be important. The basic idea that the constrained and purposeful optimizations that engineers perform routinely in the design of thermofluid flow systems can help all of us.
Better sense means a simpler, easier - to - understand, more compact, and general summary of explanations of what we see in nature. Such a summary is called a "Principle or Law".
Dr. Bejan shows that Geometric form (Shape and Structure) springs out of the struggle for better global performance subject to global and local constraints.
The thought that the same objective and constraints principle is also the mechanism that constructs geometry in natural flow systems is called "Constructal theory".
Dr. Bejan says in the preface of the book that "There are three aspects of this idea that I pursue in this book:
Better sense means a simpler, easier - to - understand, more compact, and general summary of explanations of what we see in nature. Such a summary is called a "Principle or Law".
Dr. Bejan shows that Geometric form (Shape and Structure) springs out of the struggle for better global performance subject to global and local constraints.
The thought that the same objective and constraints principle is also the mechanism that constructs geometry in natural flow systems is called "Constructal theory".
Dr. Bejan says in the preface of the book that "There are three aspects of this idea that I pursue in this book:
- To start from principle and to arrive through a mental viewing in the powerful position of predicting geometric forms that appear in nature is to practice theory. The time arrow of theory , from principle to nature, runs against the time arrow of empiricism, which begins with nature - the unexplained observation. Empiricism has been the preferred method in the study of naturally organized systems, from river and lung morphology to turbulent eddies and fractal geometry.
- The Second aspect is useful to us as engineers. Engineering is the science of systems and processes with purpose. By identifying the principle that accounts for geometric form in natural flow we improve our pwn vision as designers, as creator. For example, nature impresses us with a multitude of tree-shaped flow: lungs, vascularized tissues, river basins and deltas, lighting. botanical trees, dendritic crystals, nervous systems, street patterns and urban growth, bacterial colonies, transportation, communication and economic networks, etc. Each tree flow connects an infinity of points (volume, or area). This is beautiful example of how, in the end, the theory returns the favor to the field that created it, to engineering .
- The third aspect has to do with the role of engineering in society. once a noble and revered science (think of Leonardo da Vinci, Sadi Carnot etc.), engineering is now taken for granted. Everywhere we look, from university campus politics to the noble prize, engineering rank either low or not at all on the ladder of respect. The scientist of all time wondering about our origin. This is why Dr. Bejan believes that engineers are destined to play a role in the quest for a rational basis - a principle - for the generation of geometric form in nature.
16 - Question 12 by Kambiz Ehsani - Fluid Mechanics - 4/30/2018 - Solved
Explain what happens when we squeeze the gardening hose tip while the water is running? explain what is changing. What happens to the pressure? what happen to velocity and why? What happens to the water flow?
Solution:
Flow rate will be less due to the added friction and restriction. Velocity will be added due to less flow rate. From the Euler equation of p(term) + v(term) + z(term) = cte
We know that z term will be constant therefore when v term increase we would know that delta p will decrease and therefore p of water before exiting the hose will be increased .
Solution:
Flow rate will be less due to the added friction and restriction. Velocity will be added due to less flow rate. From the Euler equation of p(term) + v(term) + z(term) = cte
We know that z term will be constant therefore when v term increase we would know that delta p will decrease and therefore p of water before exiting the hose will be increased .
Subscribe to:
Comments (Atom)